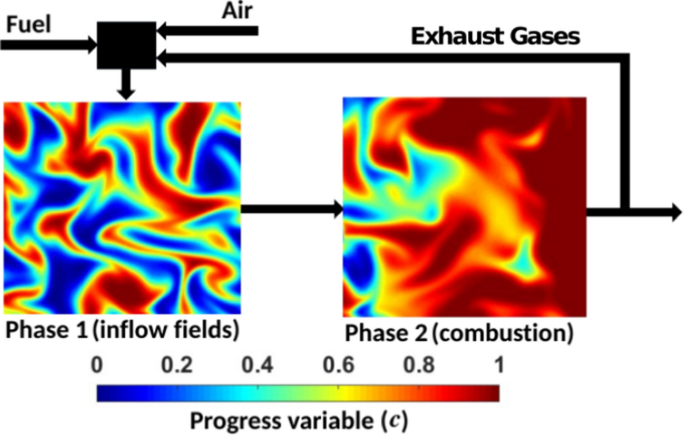
The applicability of Damköhler’s hypotheses for homogenous mixture (i.e. constant equivalence ratio) moderate or intense low-oxygen dilution (MILD) combustion processes (with methane as the fuel) has been assessed using three-dimensional direct numerical simulation data with a skeletal mechanism. Two homogeneous MILD combustion cases with different levels of \({{\text{O}}}_{2}\) concentration (4.8% and 3.5% by volume) and different turbulence intensities have been investigated to analyse the influence of dilution level, turbulence intensity and the choice of the reaction progress variable definition (i.e. different choices of major species for turbulent burning velocity and flame surface area evaluations) on the applicability of Damköhler’s hypotheses in MILD combustion. It has been found that the normalized volume-integrated burning rate remains of the same order of magnitude as that of the normalized flame surface area only for the reaction progress variable definition based on a species mass fraction which has a Lewis number close to unity (e.g. \({{\text{CH}}}_{4}\)) but the level of applicability deteriorates when the Lewis number of the species mass fraction, based on which the reaction progress variable is defined, deviates significantly from unity (e.g. \({{\text{CO}}}_{2}\)). Moreover, it has been demonstrated that the flame surface area calculation from the OH mole fraction-based information can lead to significant departures from Damköhler’s first hypothesis. It is also found that the relative magnitudes of normalised volume-integrated burning rate and normalised flame surface area are significantly affected by the level of dilution and the choice of the reaction progress variable definition. Damköhler’s second hypothesis, which provides a relation between the normalised turbulent burning velocity and the ratio of turbulent to molecular diffusivities, has been found to hold in an order of magnitude sense in homogeneous mixture MILD combustion only for the reaction progress variable definition based on species that has a Lewis number close to unity (e.g. \({{\text{CH}}}_{4}\)) but the level of disagreement increases as the Lewis number of the reaction progress variable deviates significantly from unity (e.g. \({{\text{CO}}}_{2}\)).