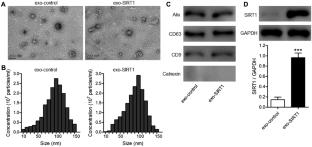
Diabetic peripheral neuropathy (DPN) is a highly prevalent diabetic complication characterized at the molecular level by mitochondrial dysfunction and deleterious oxidative damage. No effective treatments for DPN are currently available. The present study was developed to examine the impact of exosomes derived from bone marrow mesenchymal stromal cells (BMSCs) overexpressing sirtuin 1 (SIRT1) on DPN through antioxidant activity and the preservation of mitochondrial homeostasis. A DPN model was established using 20-week-old diabetic model mice (db/db). Exosomes were prepared from control BMSCs (exo-control) and BMSCs that had been transduced with a SIRT1 lentivirus (exo-SIRT1). Sensory and motor nerve conduction velocity values were measured to assess neurological function, and mechanical and thermal sensitivity were analyzed in these animals. Exo-SIRT1 preparations exhibited a high loading capacity and readily accumulated within peripheral nerves following intravenous administration, whereupon they were able to promote improved neurological recovery relative to exo-control treatment. DPN mice exhibited significantly improved nerve conduction velocity following exo-SIRT1 treatment. Relative to exo-control-treated mice, those that underwent exo-SIRT1 treatment exhibited significantly elevated TOMM20 and Nrf2/HO-1 expression, reduced MDA levels, increased GSH and SOD activity, and increased MMP. Together, these results revealed that both exo-control and exo-SIRT1 administration was sufficient to reduce the morphological and behavioral changes observed in DPN model mice, with exo-SIRT1 treatment exhibiting superior therapeutic efficacy. These data thus provide a foundation for future efforts to explore other combinations of gene therapy and exosome treatment in an effort to alleviate DPN.